The work was realised with the support of the Compagnia di San Paolo
Studying the movements of bio-molecules within the cells of the human body and the interactions they establish between them is one of the most important scientific challenges of today. Indeed, the small size of such molecules, their very high number within a cell and the resolving limits of microscopes make their observation extremely complicated. Researchers in the Molecular Microscopy and Spectrosocopy group at the IIT-Italian Institute of Technology have enhanced a technique called Fluorescence Correlation Spectroscopy (FCS), which makes it possible to obtain information on the dynamics and interactions of biomolecules without visualising them directly, but by recording changes in the intensity of the light that illuminates them while they are in motion. That is, the molecules appear as if they were fireflies in the dark, whose bright flashes are recorded. This technique provides scientists with a method for observing biological processes, even related to diseases such as tumours and neurodegenerative diseases, directly inside the cells and thus under physiological conditions.
The research was carried out by IIT in collaboration with the Politecnico di Milano and with the support of Compagnia di San Paolo. The work was published in the international journal ‘Light: Science & Applications’.
In order to be able to cure diseases such as cancer or neurodegenerative diseases, it is necessary to understand the complex mechanisms underlying cellular functioning. However, this task is hampered by the small size of the biological structures that make up any living organism. Optical microscopes are a crucial tool in biological imaging, as they help visualise cells, cell organelles and supramolecular structures such as protein complexes. Unfortunately, the resolution of a microscope is limited by physics to about 200 nanometres (500 times smaller than the thickness of a hair). The biomolecules that make up a cell are smaller than this limit.
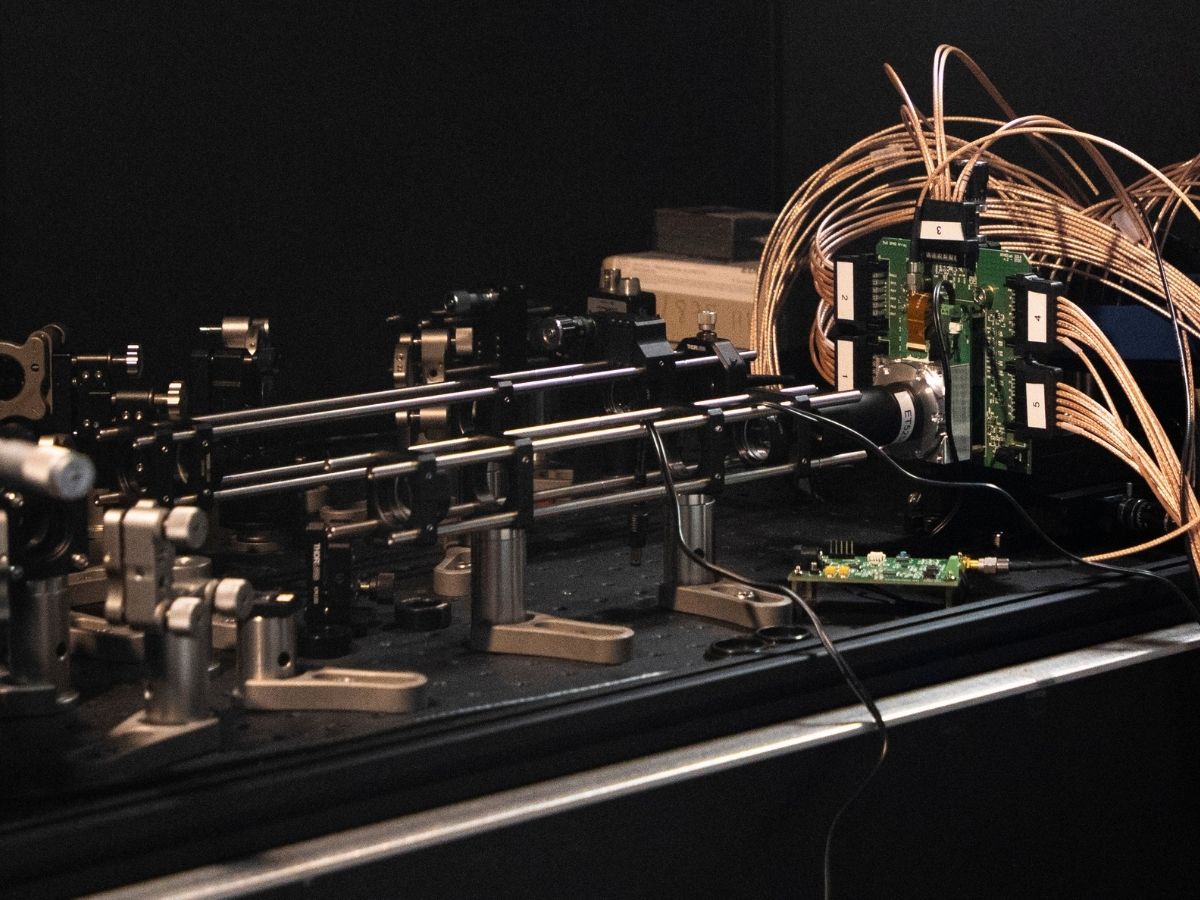
The Molecular Microscopy and Spectrosocopy group at the IIT-Italian Institute of Technology, coordinated by researcher Giuseppe Vicidomini, are working to revolutionise a technique called Fluorescence Correlation Spectroscopy (FCS), which makes it possible to obtain information on the dynamics and interactions of molecules without visualising them directly, thus circumventing the microscope’s resolution limit. The new method consists of directing a laser at a biological sample, creating a very small spot of light on it, and using a detector to record fluctuations in light intensity as the molecules move within this spot. The analysis of the fluctuations returns information on the dynamics of the molecules and their interactions.
“Taking a measurement with our method is like watching during the night a collection of fireflies flying and glowing in the dark,” says Eli Slenders, first author of the study and IIT researcher. “It’s difficult to know the positions and movements of individual fireflies in the chaos, but using a camera you can make a video where you record the flashes of light. By analysing the positions of these flashes over time we get a lot of statistical information about how fireflies move and behave in the darkness of the night.”
The technique has been in use for several decades, but researchers have introduced a particular array of light detectors that can decompose the light signal into its smallest building blocks, the photons, so that it is possible to obtain information on biomolecules that are difficult to detect with traditional sensors. Such detectors, called single-photon-avalanche-diode (SPAD) arrays, are used in many everyday technologies, such as car or mobile phone position sensors. The research group has optimised these sensors to register the light coming from individual molecules, which is several million times weaker than the light used by position sensors.
The new technique makes it possible to directly observe the processes taking place within cells, involving the interactions and structural changes of biomolecules such as DNA, RNA and proteins. This technique can be applied in genomics to visualise the life cycle of RNA; in neuroscience to understand the behaviour of certain proteins in neuronal synapses and the origin of neurodegenerative diseases; and in cancer biology to highlight specific alterations in cell division.