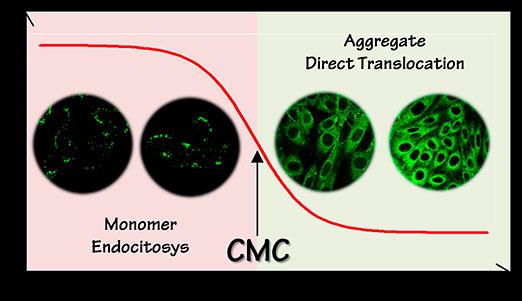
Peptides that translocate spontaneously across cell membranes have the potential to transform the field of drug delivery by enabling the transport of otherwise membrane-impermeant molecules into cells. In this regard, a 9-aminoacid-long motif (representative sequence: PLIYLRLLR, named here Translocating Motif 9, TM9) that spontaneously translocates across membranes while carrying a polar dye was recently identified by high-throughput screening, and proposed as the precursor sequence for a new generation of drug-delivery vectors. This important topic was approached at the CNI@NEST, in strict collaboration with Scuola Normale Superiore, by a multi-disciplinary team of chemists, physicists, and biologists, guided by Dr. Francesco Cardarelli (CNI@NEST). Motivated by their interest in the regulation of fundamental transport processes in living cells, the researchers designed a straightforward combination of in cuvette physico-chemical assays, rational mutagenesis, live-cell confocal imaging and fluorescence correlation spectroscopy measurements. The experiments, performed by Dr. Sara Macchi (PhD student of Scuola Normale Superiore) and colleagues, clarify the properties of this class of peptide sequences as direct-translocation vectors for delivery purposes. First, it was unveiled that the putative translocating motif TM9 does self-aggregate in a concentration-dependent manner, with self-aggregation being a necessary –yet not sufficient– step for effective membrane translocation. In fact, it is shown that membrane crossing is enabled by apolar payloads while it is completely inhibited by polar ones. It is concluded that peptide propensity to self-aggregate, exact peptide sequence, and nature of the attached cargo must be considered as crucial parameters in the effort to optimize spontaneous membrane translocation. The presented results now pave the way to the rational engineering of a new class of sequences with better tailored properties. This in turn has the potential to enable, in the next future, the direct, massive transport of drugs into target cells for therapeutic/diagnostic applications. We might envision a slightly more distant future in which researchers in the nanomedicine field can exploit such peptide sequences to rapidly probe the physiopathological state of live cells/tissues and eventually intervene in real time. Given the importance of the concepts treated, and the relevance of present results for the correct general understanding of spontaneous membrane translocation, this work was recently published in the prestigious international journal ‘Scientific Reports’ of the Nature publishing group.
Peptides that translocate spontaneously across cell membranes have the potential to transform the field of drug delivery by enabling the transport of otherwise membrane-impermeant molecules into cells. In this regard, a 9-aminoacid-long motif (representative sequence: PLIYLRLLR, named here Translocating Motif 9, TM9) that spontaneously translocates across membranes while carrying a polar dye was recently identified by high-throughput screening, and proposed as the precursor sequence for a new generation of drug-delivery vectors. This important topic was approached at the CNI@NEST, in strict collaboration with Scuola Normale Superiore, by a multi-disciplinary team of chemists, physicists, and biologists, guided by Dr. Francesco Cardarelli (CNI@NEST). Motivated by their interest in the regulation of fundamental transport processes in living cells, the researchers designed a straightforward combination of in cuvette physico-chemical assays, rational mutagenesis, live-cell confocal imaging and fluorescence correlation spectroscopy measurements. The experiments, performed by Dr. Sara Macchi (PhD student of Scuola Normale Superiore) and colleagues, clarify the properties of this class of peptide sequences as direct-translocation vectors for delivery purposes. First, it was unveiled that the putative translocating motif TM9 does self-aggregate in a concentration-dependent manner, with self-aggregation being a necessary –yet not sufficient– step for effective membrane translocation. In fact, it is shown that membrane crossing is enabled by apolar payloads while it is completely inhibited by polar ones. It is concluded that peptide propensity to self-aggregate, exact peptide sequence, and nature of the attached cargo must be considered as crucial parameters in the effort to optimize spontaneous membrane translocation. The presented results now pave the way to the rational engineering of a new class of sequences with better tailored properties. This in turn has the potential to enable, in the next future, the direct, massive transport of drugs into target cells for therapeutic/diagnostic applications. We might envision a slightly more distant future in which researchers in the nanomedicine field can exploit such peptide sequences to rapidly probe the physiopathological state of live cells/tissues and eventually intervene in real time. Given the importance of the concepts treated, and the relevance of present results for the correct general understanding of spontaneous membrane translocation, this work was recently published in the prestigious international journal ‘Scientific Reports’ of the Nature publishing group.