By Teresa Pellegrino, PI Nanomaterials for Biomedical Applications, IIT
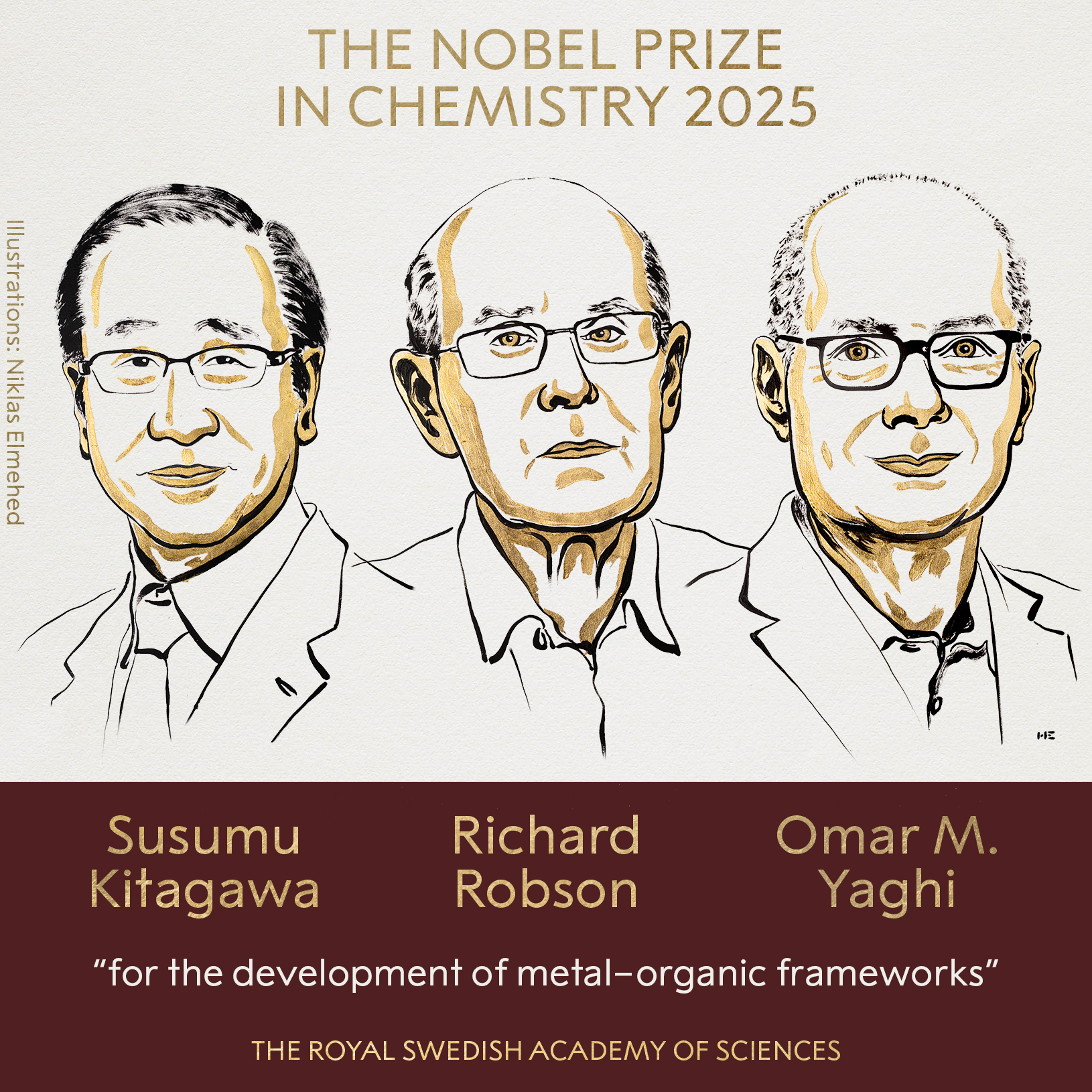
The 2025 Nobel Prize in Chemistry was awarded to Professors Susumu Kitagawa (Kyoto University, Japan), Richard Robson (University of Melbourne, Australia), and Omar M. Yaghi (University of California, Berkeley, USA) for their groundbreaking work on metal–organic frameworks (MOFs).
MOFs are solid materials full of tiny and adjustable pores, whose chemical properties can be tailored for specific purposes. They have significant potential for environmental applications, such as capturing carbon dioxide (CO₂ ) from air, harvesting water molecules from dry air to produce drinking water in arid regions, storing hydrogen (H₂), acting as catalysts to transform toxic molecules into less harmful substances.
During the Nobel Prize ceremony, the structure of MOFs was described as a “story full of holes” — because although they are made of very small solid structure, they contain vast internal space. A MOF structure as big as a small sugar cube (a couple of grams of materials) corresponded to a surface as large as a football field (small outside large inside).
The structure was also compared to a hotel, where the voids in the material are the rooms, the MOF framework is the hotel and the guest molecules like CO₂, He, or H₂O can enter and exit freely.
To help visualize the structure, a further analogy was made to wooden house frameworks. In this comparison, the metal ions act like the nodes or bolts, the organic molecules are like beams and by mounting them together highly ordered porous structures are obtained.
In the early 90s, Professor Robson was inspired by the tetrahedral structure of diamonds—where each carbon atom connects to four others in a very stable, 3D patterns. His group designed a carbon-based molecule bearing a nitrile group, which has a strong affinity for copper ions (Cu) and when mixing them, these components assembled into a tetrahedral network, mimicking the diamond lattice.
But unlike diamonds, Robson’s new material had voids—empty spaces or cavities between the building blocks. This difference was crucial. It marked the beginning of materials that were both rigid like a crystal and porous like a sponge.
Professor Kitagawa showed that gas adsorption in MOF was double: indeed, once MOFs are formed, in their dry form, they were stable enough and could adsorb gas such as methane (CH4), oxygen (O2) or nitrogen (N2).
At the same time, Professor Yaghi was looking for method to make even more stable MOFs. Started from Zn metal and oxygen atoms clusters and by adding an organic linker which contains carboxylate molecules a very porous cavities highly stable up to 300°C (which was named MOF-5) could be obtained and it contains an enormous surface area. Later, he made another conceptual contribution: changing the organic linker changes the cavity space and tunes their properties with an infinite possibility to design different MOFs provided that many organic compounds are available.
This field has evolved very fast. A myriad of structures have been already developed. To cite few examples. MOF-303 to capture air gases, CALF-2’ for capturing CO2, NU-1501 for storing H2, ZIF8 for mining rare element from waste, UiO-67 for extracting from water persistent organic pollutants such as PFAS.
As a curiosity, MOFs names seem a bit cryptic at first glance, especially with all the acronyms like HKUST-1, UiO-66, or ZIF-8. This is because for such complex structures, there is no standard and unified IUPAC name system yet developed (only for organic molecules subunits the IUPAC name exist!), therefore the MOF acronyms are named after the institution/group, for instance HKUST stays for Hong Kong University of Science and Technology, or UiO stays for University of Oslo, with some exception where the MOFs names refers to the structure, for instance ZIF stays for Zeolitic Imidazolate Framework).
These are only few examples and many more will appear. Also, beyond industrial uses, biology or other medical fields may also benefit and are fields in expansion. Indeed, biocompatibility studies and MOF applications in drug delivery, imaging and diagnosis are involving many ongoing efforts from a lot of groups worldwide. In the last few years, I had the fortune to be involved in one of those European Marie Curie International training network project called ‘HeatnMof: Heating triggered drug release from nanometric inorganic – metal-organic framework composites (https://heatnmof.eu/ )’ which aimed at developing magneto/MOF or plasmonic/MOF composites for combining hyperthermia treatment and controlled drug delivery.
As a community, a number of challenges will need to be addressed in the near future: besides providing proof of concept principles, making MOF-based process scalable and cost efficient in their specific applications ( for instance to capture CO2 or other gas from air at the minimum energy costs) and study the environmental implications of these new class of MOF materials is also among the next urgent tasks.